Reimbursement of digital therapeutics (DTx) in Switzerland
Reimbursement of digital therapeutics (DTx) in Switzerland unknown
What are DTx?
DTx open up a wide range of possibilities to support the detection and treatment of diseases for medical professionals and patients alike. For the latter, DTx can be an important aid to a self-determined, health-promoting lifestyle. DTx are therefore seen as having great potential to complement medical therapy and to enable or optimise individual prevention and aftercare.
To date, there is no definition of the term "digital therapeutics" in Swiss law. The FOPH defines digital health applications as medical devices "whose medical purpose is achieved through the main function of the digital technologies". The term thus commonly covers medical services, such as the detection, prevention or treatment of diseases, injuries and disabilities, which are mainly mediated by technologies based on (computer) hardware, software and networking. Health apps for smartphones are a common manifestation. However, browser-based web applications, software for use on traditional desktop computers or telemedicine services and digital monitoring tools (telemonitoring) also come to mind. It is irrelevant whether a DTx is used exclusively by healthcare professionals as a tool for professional practice, by patients in self-use or jointly by healthcare professionals and patients.
Typical medical areas of application for DTx include diabetology, cardiology, speech therapy, chronic nutritional disease, psychotherapy or physiotherapy.
Reimbursement by the compulsory health insurance (OKP)
In Switzerland, DTx are generally reimbursable under the current regulations of the compulsory health insurance ("OKP"). Unlike in certain other countries, the DTx reimbursement process in Switzerland takes place via the existing system for OKP services.
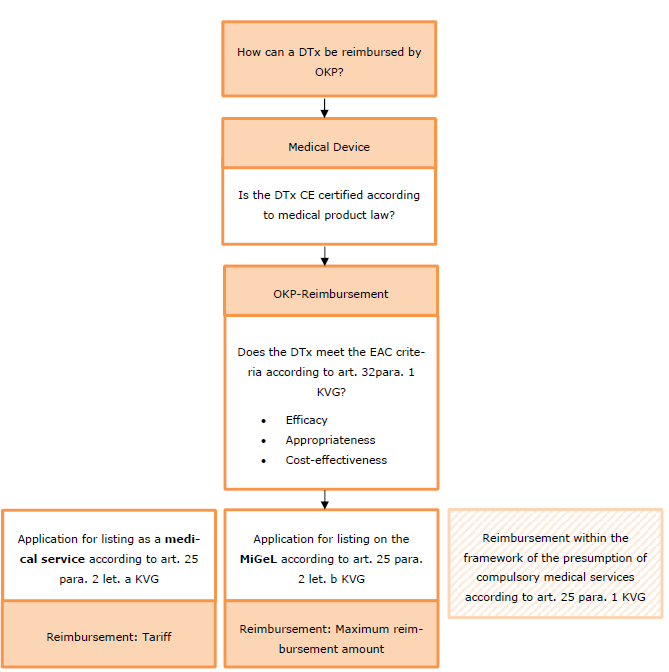
OKP application processes
Health insurance law offers various possibilities for obtaining reimbursement by the OKP for DTx or DTx services. The main options are reimbursement as a medical service, i.e. as use by medical and non-medical specialists via inclusion in the OKP list in accordance with Art. 25 para. 2 let. a KVG, or as self-use by patients or nursing professionals via inclusion in the MiGeL inaccordance with Art. 25 para. 2 let. b KVG. An application for inclusion of a medical service can be submitted to the Federal Commission "Eidgenössische Kommission für Leistungen und Grundsatzfragen" and inclusion in the MiGeL to the Federal Commission "Eidgenössischen Kommission für Analysen, Mittel und Gegenstände" using the forms provided for this purpose. A further possibility is the assumption of costs without inclusion in an OKP list based on the statutory presumption of compulsory benefits within the meaning of Art. 25 para. 1 KVG.
In order to be reimbursed under the OKP, regardless of the application process chosen, DTx must (1) comply with the requirements of medical device regulation and (2) DTx services must meet the criteria of efficacy, appropriateness and cost-effectiveness (referred to as "EAC criteria" in English or "WZW criteria" in German) of health insurance regulation. In addition, they must be assigned specific reimbursement amounts (tariffs or maximum reimbursement amounts) under health insurance law.
First requirement: marketability as a medical device
The services covered by the OKP must be medical services. Accordingly, only digital applications that serve a medical purpose can be reimbursed by the OKP. Such digital applications must always be qualified as medical devices forregulatory purposes, i.e. as products that are intended or advertised for medical use and whose main effect is not achieved by a medicinal product (art. 4 para. 1 let. b. TPA).
Accordingly, a DTx is only eligible for reimbursement under the OKP if it meets the regulatory requirements prescribed by the MedDO for the marketability of medical devices in Switzerland. This presupposes in particular that the DTx has successfully undergone a conformity assessment procedure - and thus a data protection and IT security audit.
Second prerequisite: Fulfilment of EAC criteria according to KVG
The OKP covers the costs of general sickness benefits (Art. 25 KVG), sickness care benefits (Art. 25a KVG), certain preventive medical measures (Art. 26 KVG), birth defects not covered by the disability insurance (Art. 27 KVG), for accidents (Art. 28 KVG), for maternity (Art. 29 KVG), for abortion without penalty (Art. 30 KVG) and - within narrow limits - the costs of dental treatment (Art. 31 KVG).
According to art. 32 para. 1 KVG, the central prerequisite for OKP reimbursability is that the service in question is effective, appropriate and cost-effective (EAC criteria). The EAC criteria must be fulfilled cumulatively and must be taken into account at two levels: Firstly, by the treating medical or non-medical specialist in the assessment in the specific case, and secondly, by the legislator in the designation of the services to be covered by the OKP.
Under the criterion of efficacy, themedical effect of a service is reviewed. A service is considered to be effective if (i) it is objectively suitable for working towards the intended diagnostic or therapeutic goal, (ii) a favourable ratio of benefit to harm has been demonstrated in comparison with alternative services according to scientific methods, and (iii) the transferability of the study results to Swiss clinical practice can be assumed. In general, the assessment of the efficacy of a medical technology comprises three sub-areas: Efficacy (effectiveness under study conditions), Effectiveness (effectiveness under everyday conditions in routine care) and Safety (safety).
The criterion of appropriateness examines whether a medical service can adequately achieve the desired treatment outcome in a specific individual case. The focus is thus on a favourable risk-benefit ratio. A service is appropriate if (i) it is relevant and suitable for patient care compared to alternative procedures, (ii) it is compatible with the legal conditions, ethical and social aspects or values and (iii) the quality and appropriate application in practice are guaranteed.
The costs of a medical service are decisive for cost-effectiveness. A service is economic if (i) its tariffs and prices are comprehensibly calculated, (ii) it has a favourable cost-benefit ratio in relation to the direct health care costs or the additional costs are offset by a corresponding additional benefit and (iii) the cost effects on the compulsory health care insurance are acceptable.
This blog post is based on an article by Dr. iur. et dipl. sc. nat. ETH Stefan Kohler, Attorney at Law, VISCHER AG, Zurich and BSc., MLaw Angelina Rau, Attorney at Law, VISCHER AG, Zurich, PhD Biomedical Ethics and Law, University of Zurich, on the remuneration of digital health applications (DiGA) in Switzerland, published in Life Sciences Recht, LSR Issue 1, 2023, pp. 13-22.